Microarray Testing For Plasma Cell Neoplasms
Microarray On CD138+ Plasma Cell +IGH FISH
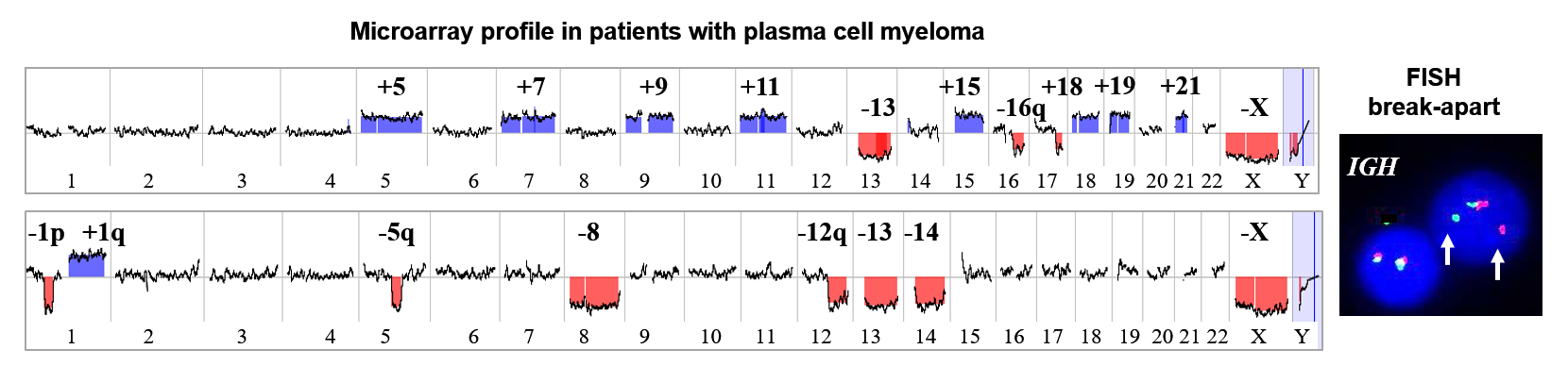 The microarray analysis performed on DNA from CD138 positive plasma cells provides additional knowledge
regarding chromosomal alterations helping in disease classification, risk stratification, and treatment
selection. In contrast to the FISH panel, microarray is high-throughput, highly accurate and superb new
tool for evaluating of cancer genomes. FISH testing for 14q32.3 (IGH) rearrangement will be automatically
performed with microarray. This microarray test replaces the former FISH panel.
The microarray analysis performed on DNA from CD138 positive plasma cells provides additional knowledge
regarding chromosomal alterations helping in disease classification, risk stratification, and treatment
selection. In contrast to the FISH panel, microarray is high-throughput, highly accurate and superb new
tool for evaluating of cancer genomes. FISH testing for 14q32.3 (IGH) rearrangement will be automatically
performed with microarray. This microarray test replaces the former FISH panel.
When ordering, please indicate a suspected or previously diagnosed plasma cell myeloma. This information is crucial for an optimal separation of CD138+ cells, which can be successfully accomplished within 72 hours upon a bone marrow sample collection.
Please see Laboratory Approaches for Testing of Patients with Plasma Cell Myeloma at the diagnosis, relapse and remission stages.
| Sample Type | CD138+ purified plasma cells isolated from a bone marrow sample, 10-20 cells are sufficient for microarray analysis |
| Indications | Suspected or definite diagnosis of Plasma Cell Myeloma, MGUS, Plasma Cell Leukemia |
| Regions Tested | Unbalanced alterations of all chromosomes |
| Advantages |
Requires very few plasma cells in a sample Detection of additional genomic alterations of prognostic and diagnostic significance |
| Limitations | Balanced rearrangements involving IGH will require concurrent FISH studies |
| FISH studies | Complementary IGH/CCND1, IGH/FGFR3, IGH/MAF, IGH/CCND3, IGH/MAFB fusion analysis in cases positive for IGH rearrangement, MYC rearrangements |
Microarray Testing For Plasma Cell Neoplasms
Microarray On CD138+ Plasma Cell +IGH FISH
The microarray analysis performed on DNA from CD138 positive plasma cells provides additional knowledge regarding chromosomal alterations helping in disease classification, risk stratification, and treatment selection. In contrast to the FISH panel, microarray is high-throughput, highly accurate and superb new tool for evaluating of cancer genomes. FISH testing for 14q32.3 (IGH) rearrangement will be automatically performed with microarray. This microarray test replaces the former FISH panel.
When ordering, please indicate a suspected or previously diagnosed plasma cell myeloma. This information is crucial for an optimal separation of CD138+ cells, which can be successfully accomplished within 72 hours upon a bone marrow sample collection.
Please see Laboratory Approaches for Testing of Patients with Plasma Cell Myeloma at the diagnosis, relapse and remission stages.
| Sample Type | CD138+ purified plasma cells isolated from a bone marrow sample, 10-20 cells are sufficient for microarray analysis |
| Indications | Suspected or definite diagnosis of Plasma Cell Myeloma, MGUS, Plasma Cell Leukemia |
| Regions Tested | Unbalanced alterations of all chromosomes |
| Advantages |
Requires very few plasma cells in a sample Detection of additional genomic alterations of prognostic and diagnostic significance |
| Limitations | Balanced rearrangements involving IGH will require concurrent FISH studies |
| FISH studies | Complementary IGH/CCND1, IGH/FGFR3, IGH/MAF, IGH/CCND3, IGH/MAFB fusion analysis in cases positive for IGH rearrangement, MYC rearrangements |